 Boulder iQ is an expert contract consulting firm providing all the services a life sciences company needs to get its product to market.
Boulder iQ is an expert contract consulting firm providing all the services a life sciences company needs to get its product to market.
The single-source solution speeds the product development and regulatory submissions process.

Since 2009, Boulder iQ experts have helped companies navigate the process of getting medical device, diagnostic, and combination products to the market quickly.
- Regulatory Affairs
- Quality Assurance
- Design
- Engineering
- Manufacturing
- Packaging
- Sterilization

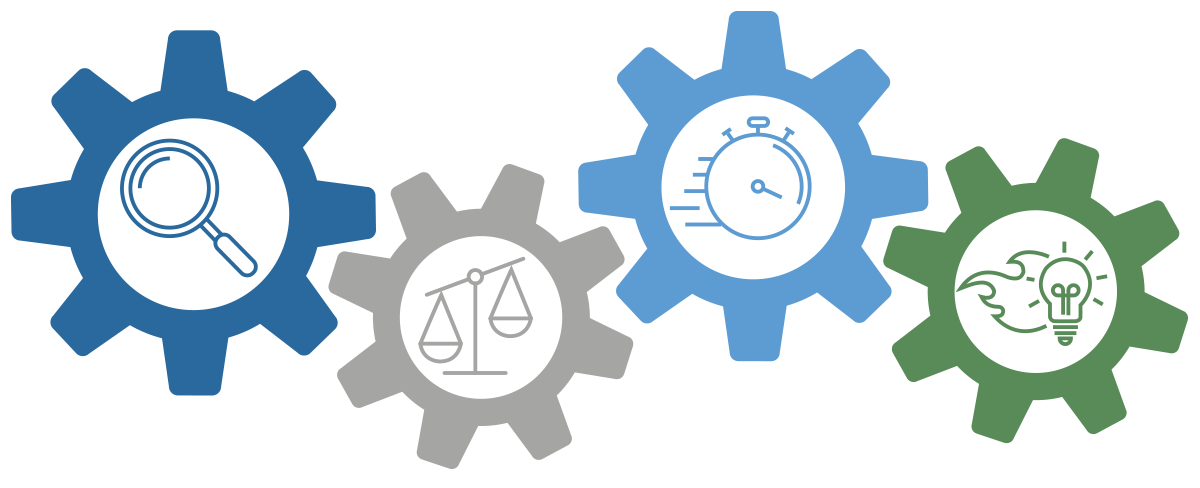
The Boulder iQ Approach
![]()
Understand customer needs
Gaining a deep understanding of user needs at the beginning of the development process – starting with early drafting of instructions for use – results in time and cost savings, and the chance to introduce innovative products in advance of the competition.
![]()
Know the difference between wants and needs
Boulder iQ helps clients prioritize early in the process. Users walk through how they will implement the product, and developers gain perspective and details about how their device will achieve key goals – all before expending substantial time and money in development.
![]()
Move beyond phase-gate project management
Traditional QMS policy dictates that significant work on given phases of product development begin only when the requirements of the previous phase have been met. By taking a fluid approach that eliminates the strict “gates,” Boulder iQ can reduce time to market 10-60%.
![]()
Think smart
By changing the order of selected steps in the development process, Boulder iQ helps clients maintain laser focus on the end result instead of each phase. Thinking smart never skips a step. Instead, it speeds time to market within a controlled process.
Our Facility
![]()
Boulder Regulatory Affairs and Quality Assurance operates within Boulder iQ’s two facilities. The buildings include 20,000 square feet in foothills of the Rocky mountains in Boulder, Colorado.
- Largest CD sterilizer in the world
- Certified ISO Class 7 (Class 10,000) 1,000-square-foot clean room
- Inspection and test lab
- Client-customized wet lab
- Machine shop
- 3D print shop
- Client-customized test set-ups
- Customizable fume hoods


