
Think Fast! Challenging the Phase-Gate Paradigm to Improve Time to Market
THE REGIMENTED PARADIGM
Medical device development is a regulated activity, and rightfully so. The provisions of the FDA Quality System Regulation, international standard ISO 13485 and similar oversight regulations have significantly improved the quality of medical device designs.
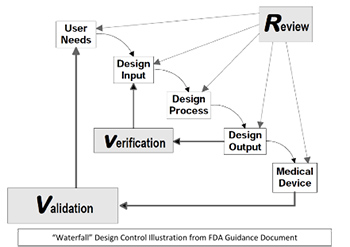 They require a Quality Management System (QMS) be established by the developer and in most cases the use of Design Controls along with specific documentation and review requirements throughout the process, including a Design History File (DHF), evidence of Verification and Validation (V&V) testing and a Device Master Record (DMR) on the release of the design to manufacturing. A Design Control process diagram is shown below, excerpted from the FDA Guidance Document “Design Control Guidance for Medical Device Manufacturers.”
They require a Quality Management System (QMS) be established by the developer and in most cases the use of Design Controls along with specific documentation and review requirements throughout the process, including a Design History File (DHF), evidence of Verification and Validation (V&V) testing and a Device Master Record (DMR) on the release of the design to manufacturing. A Design Control process diagram is shown below, excerpted from the FDA Guidance Document “Design Control Guidance for Medical Device Manufacturers.”
As part of their QMS, most companies include a “phased” product development process, with specific requirements for each phase. The compliance of meeting the requirements of each phase is strictly enforced by QMS quality policies requiring documented design reviews and phase reviews before completing the DHF for each phase of the process. In many cases, these reviews become the gatekeepers, allowing significant work on the following phase to begin only when the DHF requirements of the previous phase have been satisfied, thus creating a phase-gate approach to controlling the progress of medical device development.
Many tools are available to track activities through the development phases such as Microsoft Project, Playbook, Smartsheet, Agile, etc. But these tools only help implement a specific way of thinking, of proceeding along the development path. We know that significant advantages can be achieved by challenging traditional thinking, without loss of quality control and in full regulatory compliance.
There’s nothing inherently wrong with establishing product development phases. It’s an appropriate way to set up a project. We’ve found ways, however, to make dramatic improvements to overall program efficiency while still maintaining project phase controls.
THINK FAST!
Time to market is a critical factor in business success. Once a new product has been conceived, every month, every week it is delayed from entering the marketplace represents lost revenue and presents competitive advantages to others. So how can we improve time to market within a controlled process?
Do everything that is doable up front!
Just because development of V&V test protocols isn’t listed until Phase 3 of the development process, doesn’t mean they can’t be drafted in Phase 1 as soon as the product requirements have been drafted. The same is true for other procedures and documents. Will they change? Of course! They’ll need to be refined as additional information is obtained and details determined. But there are significant advantages to doing as much as possible as soon as possible as described below.
Write the IFU first!
In most systems, writing the Instructions For Use (IFU) document for a device is done toward the end of the development process when all the pertinent details are known. We’ve found great advantage of drafting the IFU as part of the Formative Usability Study (per ISO 62366). The formative usability study typically requires document sketches and presentation of concepts to potential users to confirm that the approach is sound. We go beyond that. In addition to the illustrations, we present an in-service training session to the users following the draft IFU. This is an eye-opener! It really allows the potential user to walk through how the device will be used, but also allows the developers to think in detail about what they’re trying to achieve for their product users. Up front!
This up-front IFU approach has a dramatic impact on the accuracy of the product requirements and specifications. Accordingly, it reduces the number and depth of iterations during the design and testing processes.
Information from the IFU, as modified during the formative usability study, the resulting product requirements document and specifications can then be used to outline test protocols for engineering performance confirmation, verification, and validation to be performed in subsequent phases. Knowing how the design will be tested very early in the process helps streamline the design activities.
Following this Think Fast approach, develop the table of contents for the DHF and for the 510(k) up front. Make an electronic (and physical if desired) file folder for each item in these tables of contents and begin populating them with draft documents as early as possible. Then, as the later phases are completed, there’s less time effort needed to complete the DHF and the technical file portions of the 510(k) submittal. They’re already mostly done! Think Fast!
Meeting the Documentation and Design Control Requirements
The Think Fast approach doesn’t skip anything required. It simply eliminates the “gate” from traditional phase-gate thinking. To complete any given phase, review meetings are held to confirm that all documents required for that phase have been reviewed and approved appropriately. Fine. That doesn’t mean that you can’t already have a large number of documents drafted for future phases, already for final details to be added then. And by doing those documents early, you force yourself to think about what each will require, thus avoiding oversights that have to be filled in later.
There’s a common objection to writing a document, test procedure, or process instruction before the data is ready because “I’ll just have to re-do it later.” No very much, we’ve found. Once the IFU, requirements and specifications are in place, the majority of down-stream documents can be drafted with blanks for the details to be filled in later, with all the attendant benefits previously mentioned.
What about templates? Taking the Think Fast approach a step further, can I develop templates to be used in virtually any medical device development project? The answer is yes, to a point.
Each project, each product has its own unique characteristics. Product requirements and specifications for an IV pump and its tubing set are drastically different from those of an electrosurgical generator and its disposable surgical pencils. So, templates tend to be limited, but can still have some value in establishing the major items to be completed.
And what about use of Microsoft Project, Agile approaches, etc.? Those and similar tools can be used with the Think Fast approach as well. The tasks and sprints are restructured to include early implementation of the IFU, test procedures, DHF and 510(k) submittal document structures and so forth. It’s thinking about fast, more efficient product development that’s the key to achieving best time to market while maintaining all the appropriate quality requirements and simultaneously complying with all pertinent regulations.
CONCLUSION
Efficient design depends on understanding the breadth of the user needs and testing requirements for the product at the very beginning. Knowing these also allows early drafting of the key documents that will be required in later phases of the project. Think Fast refers to thinking in ways specifically targeted to reducing time to market while maintaining quality and compliance. To Think Fast is also to Thing Smart! The result is time and cost savings in the development process and introducing innovative new products in advance of the competition. It’s just good business!
Jim Kasic, President and CEO, Boulder iQ
Peggy Fasano, former Chief Operating Officer, Boulder iQ
